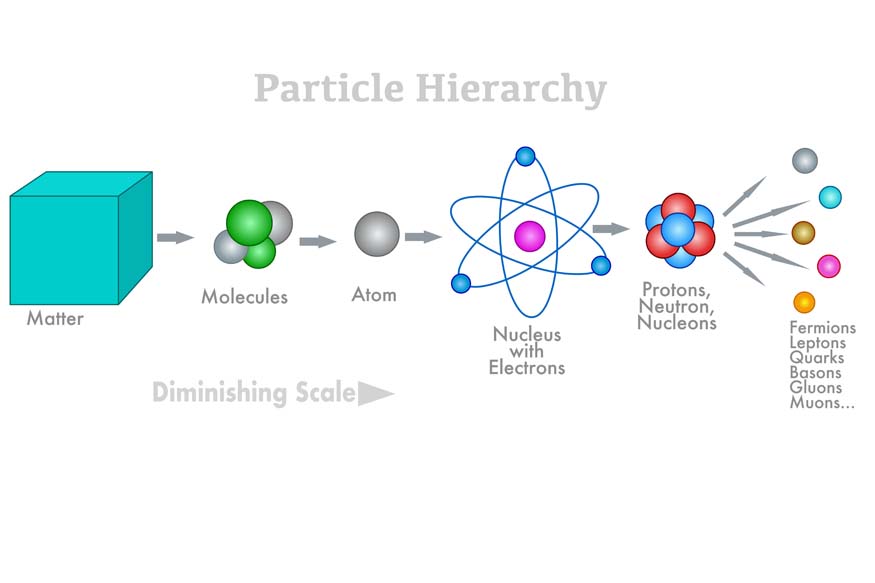
Matter is a substance that makes up all physical objects in the universe. From the tiniest speck of dust to the largest celestial bodies, everything is composed of matter. But what exactly is matter made of? To understand this, we must delve into the fundamental building blocks that form the core of our existence. This article will take us through the world of atoms, molecules, and the subatomic particles that constitute them.
Atoms: The Basic Unit of Matter
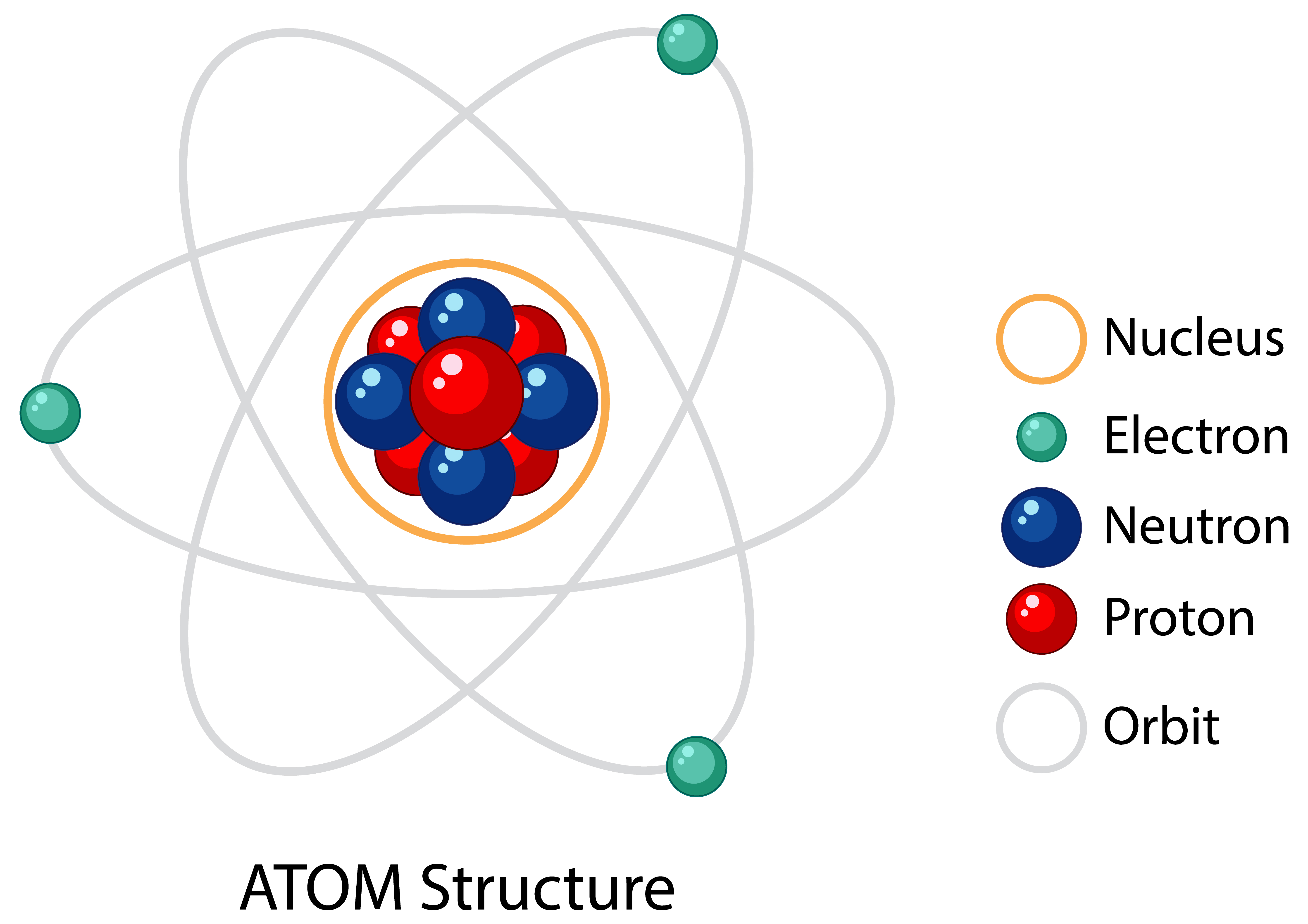
Atoms are the smallest units of matter that have the identity of an element. The concept of the atom dates back to ancient Greece, but a proper pavement for atomic theory was not laid until the 19th and 20th centuries scientists contributed to it. These renowned scientists, namely John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, have become the pillars of modern atomic theory.
An atom consists of a nucleus, which has protons and neutrons within, surrounded by a cloud of electrons. Protons have a positive charge, Electrons are negatively charged, and Neutrons, as their name suggests, are neutral with no charge. The number of protons in the nucleus, known as the atomic number, determines the element to which the atom belongs. For example, all atoms with 2 protons are helium atoms, while those with 8 protons are oxygen atoms.
Subatomic Particles: Protons, Neutrons, and Electrons
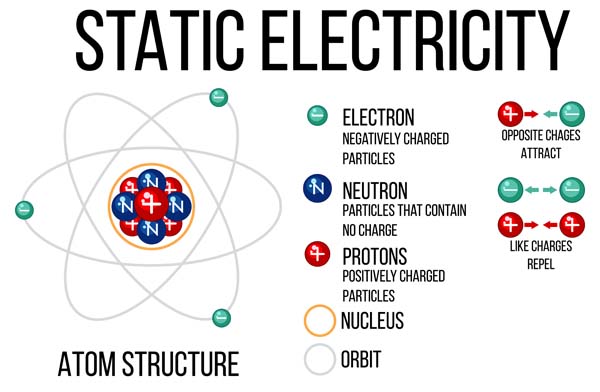
Protons: Protons are one of the key components of an atom’s nucleus. They carry a positive charge and have a relatively large mass compared to electrons. The number of protons in an atom determines the element and its place on the periodic table. For instance, lithium always has 3 protons.
Neutrons: Neutrons are neutrally charged or no-charge particles found in the nucleus alongside protons. Their mass is similar to the protons but does not carry an electric charge. Neutrons play a crucial role in adding mass to the atom and stabilising the nucleus. Variations in the number of neutrons in an atom lead to different isotopes of the same element.
Electrons: Electrons carry a negative charge and orbit the nucleus in various energy levels or shells. Despite their tiny mass, electrons are essential for chemical bonding and reactions. The arrangement of electrons around the nucleus determines the chemical properties of an element and its ability to form compounds.
Quarks and Gluons: The Inner World of Protons and Neutrons
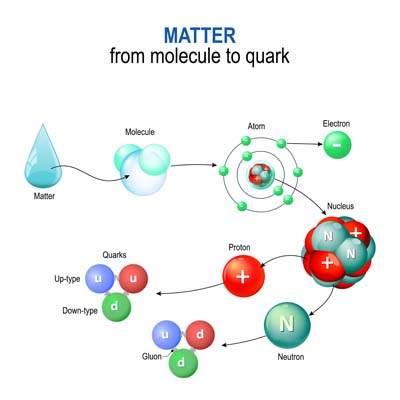
When we deep dive into knowing the nucleus, we will find that protons and neutrons are not elementary particles. They are made up of even smaller particles called quarks, which are held together by particles known as gluons.
Quarks: Quarks are fundamental particles with six different types or “flavours”: up, down, charm, strange, top, and bottom. Protons comprise two up quarks and one down quark, while neutrons consist of two down quarks and one up quark. Quarks are never found alone; they are always confined within larger particles like protons and neutrons due to the strong nuclear force.
Gluons: Gluons are the force-carrying particles that bind quarks together. They mediate the strong nuclear force, one of the four fundamental forces of nature, which is responsible for holding the atomic nucleus together. Without gluons, quarks would not form protons and neutrons, and atoms as we know them would not exist.
Molecules: The Building Blocks of Compounds
Atoms combine to form molecules, which are the building blocks of compounds. A molecule consists of two or more atoms bonded together through chemical bonds. These bonds can be covalent, ionic, or metallic, each with distinct characteristics.
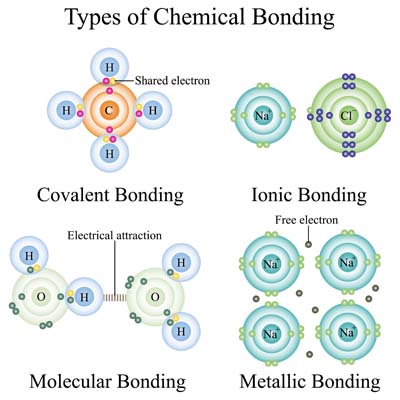
Covalent Bonds: Atoms share electrons to achieve a stable electron configuration called a Covalent Bond. This type of bond is common in organic molecules, where carbon atoms form the backbone of complex structures. Water (H₂O) and carbon dioxide (CO₂) are appropriate examples of molecules with covalent bonds.
Ionic Bonds: Ionic bonds are formed when electrons are transferred from one atom to another, resulting in oppositely charged ions that attract each other. This type of bond is usually found in salts like sodium chloride (NaCl), where sodium gives an electron to chlorine, forming a bond between the resulting ions.
Metallic Bonds: Metallic bonds occur in metals, where atoms share a “sea” of electrons that flow freely around a lattice of positively charged ions. This bond gives metals their characteristic properties, such as electrical conductivity and malleability.
The Periodic Table: Organising the Elements
The periodic table has elements arranged systematically based on their atomic number, electron configuration, and recurring chemical properties. The periodic table was developed by Dmitri Mendeleev in the 19th century, which allows scientists to predict the behaviour of elements and their compounds.
Groups: Elements in the same vertical column, known as a group, have similar chemical properties. For example, the elements in Group 1 (alkali metals) are highly reactive and form strong bases when combined with water.
Periods: Elements in the same horizontal row, called a period, have the same number of electron shells. As you move across a period, the properties of elements change gradually due to the increasing number of protons and electrons.
Blocks: The periodic table is also divided into blocks namely s, p, d, and f based on the electron orbitals being filled. This division helps understand the structure and bonding behaviour of the elements.
Conclusion
Understanding the essential components of matter is vital for students starting their exploration of the sciences. From the subatomic particles that have atoms to the molecules they create, each element is integral to the structure of this universe. The periodic table acts as a framework, where elements are arranged systematically to highlight patterns and characteristics crucial to the study of chemistry and physics.
For more such informative/interesting blogs, visit Center Point School.