Atoms are the fundamental building blocks of matter. Everything around us, from the air we breathe to the devices we use, comprises atoms. Despite their tiny size, atoms have a complex structure that plays a crucial role in the properties and behaviours of different substances. To understand chemistry and the natural world, it is essential to grasp the basic components of an atom: protons, neutrons, and electrons. Let us delve into the fascinating world of atomic structure and explore these components in detail.
The Basics of an Atom
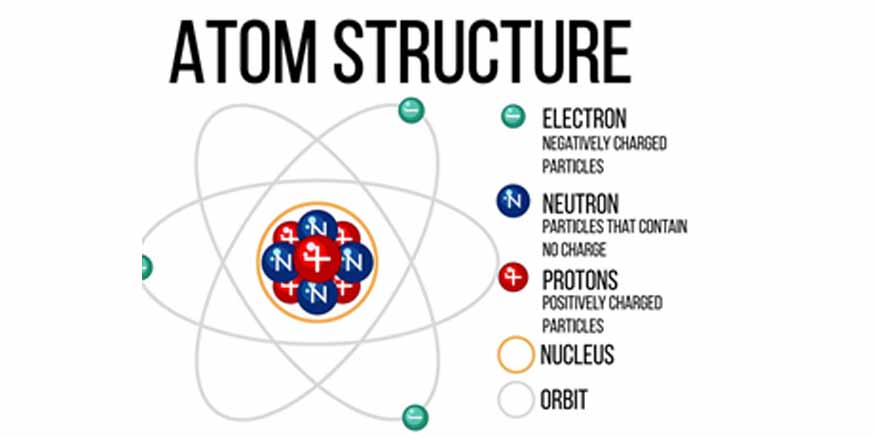
An atom consists of a nucleus at its centre surrounded by a cloud of electrons. The nucleus contains two types of subatomic particles namely protons and neutrons. Electrons revolve around the nucleus in various energy levels known as electron shells. The number and arrangement of these subatomic particles determine the identity and characteristics of an element.
Protons
Protons are positively charged particles located in the nucleus of an atom. Each proton carries a positive charge. The atomic number of an element is determined by the count of protons present in the nucleus. For example, hydrogen has one proton, while carbon has six. Each element has a unique atomic number and the periodic table is organised based on this.
Protons also contribute to the mass of an atom. Although they are significantly smaller than neutrons, their positive charge is vital in holding the electrons in orbit through electrostatic attraction. This force ensures electrons do not fly away from the nucleus.
Neutrons
These electrically neutral particles exist in the nucleus alongside protons. They have no charge but contribute significantly to the atom’s mass. Isotopes of an element occur based on the number of neutrons in the nucleus. Isotopes are variants of elements that have the same number of protons but different numbers of neutrons.
Neutrons play a crucial role in stabilising the nucleus. The positive charge of protons creates a repulsive force that would push them apart. Neutrons help to mitigate this repulsion by providing an additional attractive force, known as the nuclear force, which holds the nucleus together.
Electrons
Electrons are negatively charged particles that orbit the nucleus in defined energy levels or shells. The number of electrons in a neutral atom is equal to that of protons, to balance the positive charge.
The chemical properties and reactivity of an atom are determined by the arrangement of electrons in it. Electrons occupy energy levels, starting with lower levels filled first moving towards the higher levels. The distribution of electrons in these shells follows a set of rules known as the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. These rules dictate how electrons fill the available orbitals and influence the atom’s behaviour in chemical reactions.
Facts:
- Atoms are mostly empty space. If an atom were the size of a football stadium, the nucleus would be like a tiny pea in the centre!
- When you rub a balloon on your hair, you’re transferring electrons, which gives the balloon a static charge and makes it stick to things.
The Electron Cloud
Neutrons, unlike protons, are confined to the nucleus and, electrons exist in a “cloud” around the nucleus. This cloud represents regions of space where electrons are likely to be found. These regions called orbitals can hold a specific number of electrons in them. The shape and size of orbitals vary depending on the energy level and the presence of other electrons.
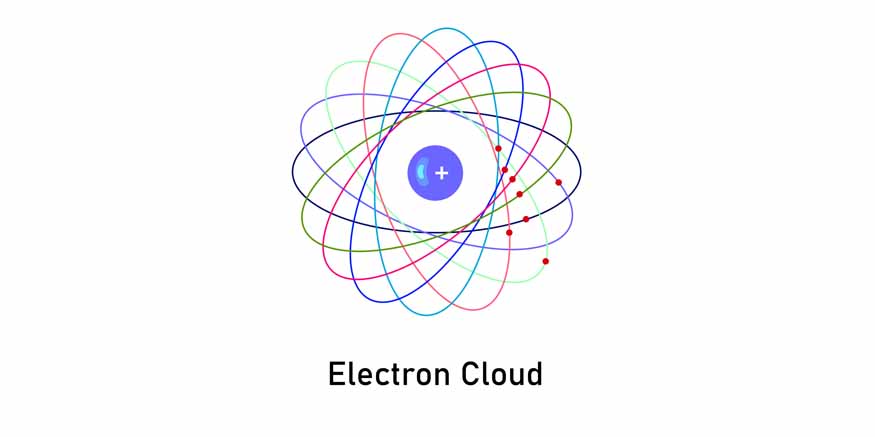
Electron clouds challenge the traditional view of electrons as particles orbiting the nucleus in fixed paths. But quantum mechanics describes electrons as existing in a state of probability, where their exact location cannot be precisely determined. This probabilistic nature is a fundamental aspect of the behaviour of subatomic particles.
Atomic Mass and Isotopes
The combined number of protons and neutrons in the nucleus determines the atomic mass of an element. Since electrons have a negligible mass, they don’t significantly contribute to the atomic mass. For instance, carbon-12, the most common isotope of carbon, has six protons and six neutrons, giving it an atomic mass of 12 atomic mass units (AMU).
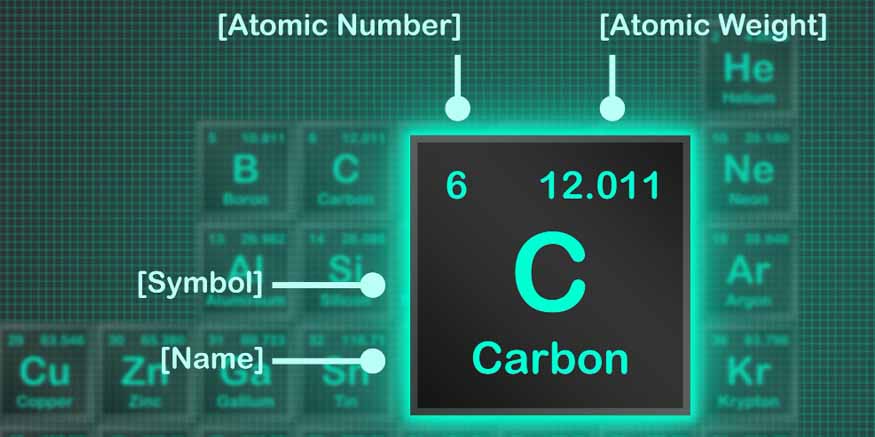
Isotopes of an element have the same number of protons but different numbers of neutrons. This variation in neutron count leads to differences in atomic mass. For example, carbon-14, another isotope of carbon, has six protons and eight neutrons, resulting in an atomic mass of 14 amu. Isotopes have important applications in medicine, archaeology, and environmental science.
Conclusion
Understanding the structure of an atom is fundamental to grasping the principles of chemistry and physics. Protons, neutrons, and electrons each play distinct and vital roles in defining the properties and behaviours of elements. The arrangement of these subatomic particles within the atom influences everything from the stability of the nucleus to the chemical reactivity of that element. By exploring these building blocks of matter, we gain insights into the underlying principles that govern the natural world.
As we continue to delve deeper into the mysteries of atomic structure, we unlock new possibilities and advancements in science and technology. The study of atoms not only enriches our knowledge of the universe but also drives innovation and progress in various fields.
For more such informative/interesting blogs, visit Center Point School.