Acids, bases, and salts are fundamental components in chemistry and play vital roles in our everyday lives. Whether it is the tangy taste of lemon juice, the slippery feel of soap, or the table salt we sprinkle on our food, these substances are all around us. Now, let us explore the world of acids, bases, and salts along with a few fascinating experiments to raise your curiosity.
Acids
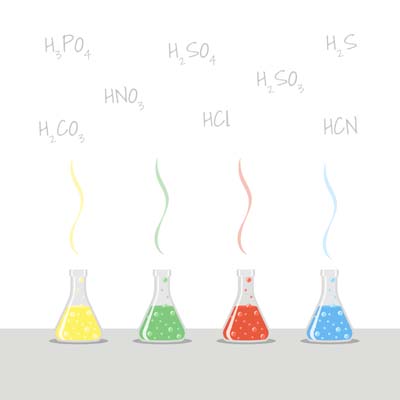
Acids are special chemicals that release hydrogen ions (H⁺) when mixed with water. You might find acids in everyday items like citrus fruits, vinegar, and even in some fermented foods. Some of the commonly known acids are Hydrochloric acid (HCl), Sulfuric acid (H2SO4) and Acetic acid (HC2H3O2).
Types of Acids
Organic Acids: These acids come from nature directly. The citric acid in lemons and the acetic acid in vinegar are good examples.
Inorganic Acids: These acids are man-made by following proper procedures. Sulfuric acid (H2SO4) is a good example, which is used in many fertiliser production.
Cool Facts About Acids
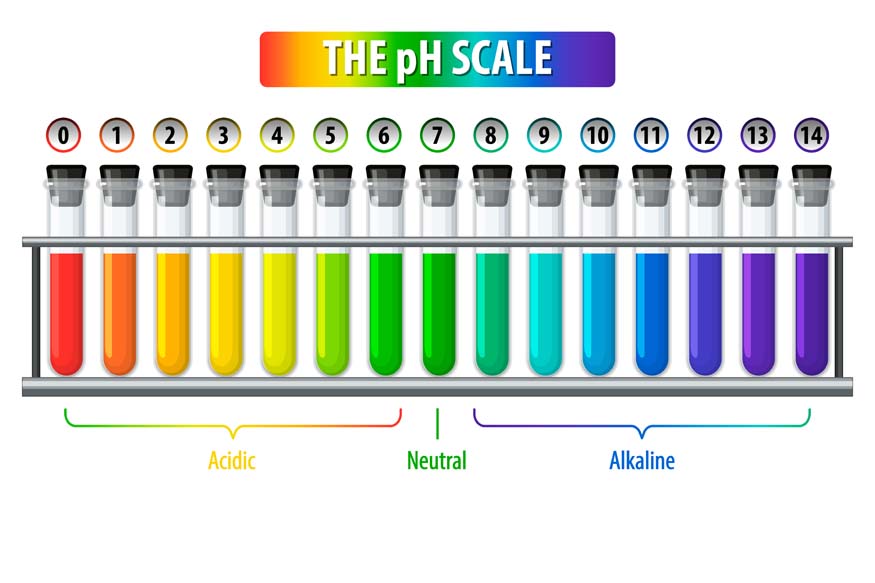
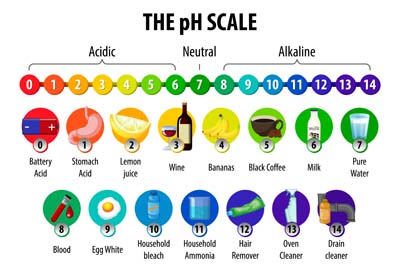
- pH Scale: Acids are measured on a scale from 0 to 14. If the pH of a substance is less than 7, it is termed acidic.
- Indicators: Acids can alter the colour of certain indicators. For example, litmus paper turns red when it comes into contact with an acid.
- Physical Properties of Acids
- Taste and Smell: Acids give off a strong smell and have a sour taste in general.
- Boiling Point: Acids often have higher boiling points than water, meaning they need more heat to turn into gas.
- Electricity: Acids can conduct electricity in a solution due to their ability to release charged particles known as ions.
Chemical Properties of Acid
Acid has various chemical properties when they come in contact with different substances. One of the chemical properties of acids include –
Reaction with metal: When an acid reacts with a metal, it produces hydrogen gas and the corresponding salt.
Metal + Acid → Salt + Hydrogen
Example: When hydrochloric acid combines with zinc metal, it produces hydrogen gas and zinc chloride.
Zn + 2HCl → ZnCl2 + H2
Base
Base is a chemical compound that produces salts and hydroxide ions (OH–) in water. Examples include Potassium hydroxide (KOH), Calcium hydroxide (Ca(OH)2), and Sodium hydroxide (NaOH).
Physical Properties of Base
Base has distinct physical properties that make them easy to identify. They typically exhibit a bitter flavour and have a slippery or soapy texture. When a base is tested with red litmus paper, it turns blue. They conduct electricity when dissolved in water due to the availability of free ions in the solution.
Chemical Properties of Base
Reaction with Metals: Alkali (base) produces salt and hydrogen gas when it reacts with metal.
Alkali + Metal → Salt + Hydrogen
Example: When potassium hydroxide reacts with zinc, it forms potassium zincate and hydrogen gas.
The chemical equation for this reaction is:
Zn+2KOH+2H2O→K2Zn(OH)4+H2
Salts
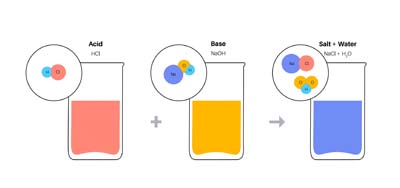
When an acid and a base react, they form salts. These are neutral substances with no electrical charge. Sodium chloride(NaCl), commonly known as table salt, is the most familiar type of salt known to many people. NaCl is used in cooking to enhance the flavour of food.
Key Points About Salts:
- Composition: Salts are made of ions held together by electrostatic attraction, and the bond formed is strong.
- Forms: Salts can be crystalline, transparent or opaque, and are in many colours.
- Solubility: Most salts dissolve in water and can conduct electricity.
- Taste: These salts can taste salty, sour, sweet, bitter, or savoury (umami).
- Uses: Salts are used in cooking, preservation, industry, and health.
Physical Properties:
- Crystalline Nature: Salts like table salt often form crystals. Two commonly known crystalline salts are Washing soda: Sodium carbonate decahydrate (NaCO3.10HO), and Gypsum: Calcium sulfate (CaSO4.2HO).
- Conductivity: Salts break down into ions when mixed with water and act as a good conductor of electricity.
- No Odour: Salt, by itself, has no smell because it is made up of particles that do not easily evaporate and release any strong scents.
Facts and Fun Experiments
Did You Know?
Natural Indicators: Some plants can act as natural pH indicators. For instance, red cabbage contains a pigment called anthocyanin that changes colour when it is in contact with an acidic or basic solution. Boil red cabbage and use the resulting juice to test the pH of different household substances!
Fun Experiments:
Invisible Ink: You can create invisible ink with lemon juice as it is acidic. Write a message on paper using lemon juice, let it dry and place the paper near a light bulb (with an adult’s help). The bulb’s heat will turn the lemon juice brown, showing your secret message!
Fizzing Reaction: Creating a fun fizzing reaction using baking soda (a base) and vinegar (an acid). When these two substances mix, they release carbon dioxide gas, which creates bubbles showing the fizzy reaction. Try adding food colour to it and watch the colourful twist!
Homemade pH Indicator: As mentioned earlier, you can use red cabbage juice to test the pH of various substances. Simply blend or boil some red cabbage, and strain the liquid. Now use this liquid to test the pH of daily household substances like soda, baking soda, vinegar, and soap. Watch as the colour changes to indicate whether the substance is acidic or basic!
Conclusion
Understanding acids, bases, and salts is essential for every student. These substances are crucial in scientific studies and play significant roles in everyday life. Acids and bases are involved in numerous chemical reactions, and their interactions often result in the formation of salts. This knowledge helps students appreciate the chemical nature of the world around them and lays the foundation for further studies in chemistry and related fields.
For more such informative/interesting blogs, visit Center Point School.