Have you ever wondered why objects float or sink in water? This interesting phenomenon is determined by the factor called Density that determines whether an object will float or sink. In this article, we will dive deep into the concept of density and uncover the scientific principles behind why objects behave differently in water.
Understanding Density
What is Density?
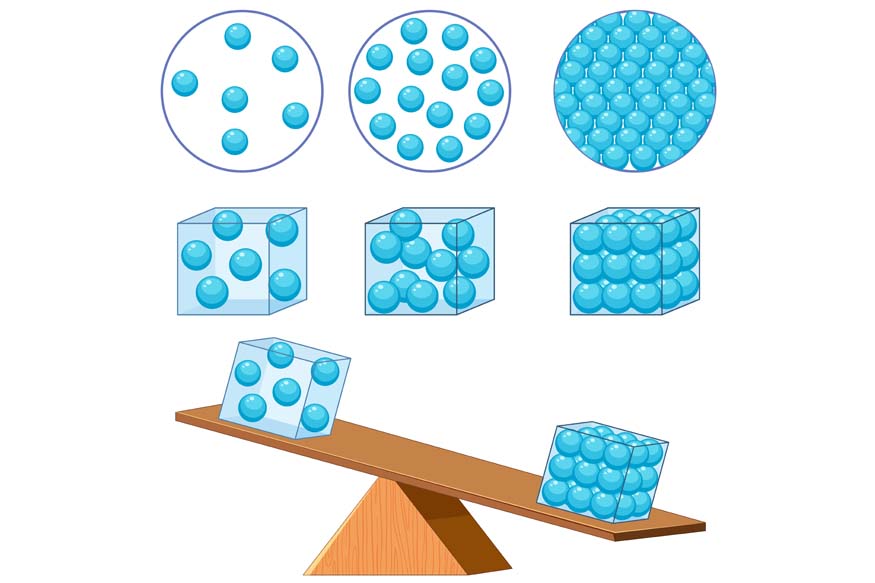
Density is defined as the mass of an object per unit volume. In simpler terms, it is the measure that determines how much matter is packed in a given space. The formula for density is:
Density = Mass / Volume
The density of a substance can be influenced by various factors, including its composition and the arrangement of its molecules. Denser materials have molecules packed closely together, while less dense materials have molecules spread apart.
The Principle of Buoyancy
To understand why objects float or sink, we need to understand the principle of buoyancy. Buoyancy refers to the upward force exerted by a fluid (like water) on an object placed in it. This force is crucial in determining whether an object will float or sink.
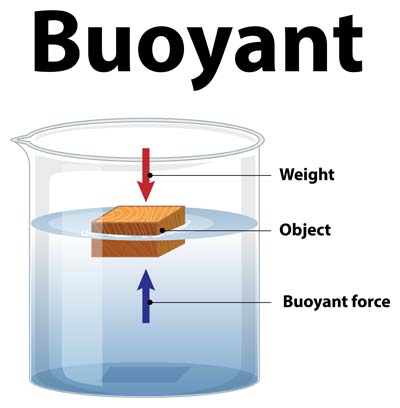
According to Archimedes’ principle, any object either fully or partially submerged in a fluid gets pushed up by a force equal to the weight of the fluid it displaces. This is why you might notice that some objects feel lighter when they’re in water, and it also explains why they either float or sink based on the amount of water they displace.

When you place an object in water, it pushes the water aside, causing a volume of water to be displaced. The weight of this displaced water creates an upward force on the object. The object will float if this force is equal to or greater than the object’s weight, and will sink if the force is less.
Floating and Sinking: Density of Matter
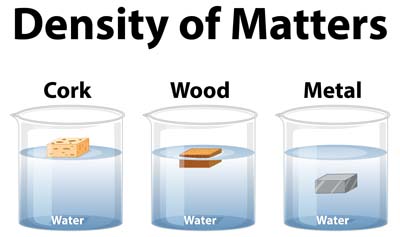
Now, let us connect density with buoyancy. When an object is placed in water, two forces come into play: the object’s weight (a downward force) and the buoyant force (an upward force).
Floating: An object with a lower density than water will displace a volume of water that weighs the same as or more than the object itself. Consequently, the buoyant force generated will be sufficient to balance the weight of the object, allowing it to float. For instance, wood floats due to its density being less than water.
Sinking: If an object has a density greater than water, it will displace a volume of water that weighs less than the object itself. Consequently, the buoyant force will not be adequate to counteract the weight of the object, leading it to sink. A common example of this phenomenon is a metal coin, which sinks due to its higher density compared to water.
Real-World Applications
Understanding density and buoyancy has practical applications in various fields.
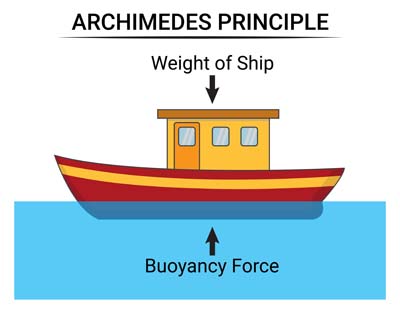
- In engineering, ships and submarines are designed with these principles in mind to ensure they can float or submerge as needed.
- In the medical field, understanding the density of different body tissues helps in imaging techniques such as MRI and CT scans.
Floating and Sinking Objects
Here is the table with 10 examples of floating and sinking objects:
|
Serial Number |
Floating Objects |
Sinking Objects |
|
1 |
Wood |
Metal Coin |
|
2 |
Plastic Bottle |
Stone |
|
3 |
Cork |
Iron Nail |
|
4 |
Apple |
Brick |
|
5 |
Ice |
Marble |
|
6 |
Rubber Duck |
Scissors |
|
7 |
Ping Pong Ball |
Key |
|
8 |
Styrofoam Cup |
Spoon |
|
9 |
Feather |
Paperweight |
|
10 |
Leaf |
Battery |
Interesting Facts on Density
You can alter the density of a substance by heating, cooling, or adding something to it. If an object sinks in water, it indicates that its density is greater than water. There are two ways to make that object float:
- Increase the density of the water so it becomes denser than the object. For instance, an egg usually sinks in plain water because it is denser. By adding salt to the water, you increase its density, allowing the egg to float. This method also works for people, but you need a lot of salt—like in the ocean or the Dead Sea!
- Increase the volume of the object so it becomes less dense than the water. A good example is ice floating on water. Ice forms when water freezes, causing it to expand as the molecules spread out to create the ice structure. Since ice is less dense than water, it floats. This also explains why ships float, even though they are made of steel. Ships are designed to have a lot of open space, which allows them to displace more water than their weight, enabling them to float.
Conclusion
Density plays a crucial role in deciding if objects will float or sink. By looking at how an object’s density relates to the buoyant force from a fluid, we can understand why some things float while others sink. This understanding is interesting and even has significant uses in science, engineering, and daily life. The next time you observe something floating or sinking, you’ll grasp the science behind it!
For more such informative/interesting blogs, visit Center Point School.